Clinical laboratories provide the foundation for modern diagnostics and patient care, yet their reimbursement under Medicare has been under sustained pressure since the Protecting Access to Medicare Act (PAMA) of 2014. While Congress intended PAMA to tie Medicare payment rates for laboratory services to the broader private market, flaws in its implementation have caused significant underpayment for many labs. With new cuts scheduled to take effect in 2026, the Reforming and Enhancing Sustainable Updates to Laboratory Testing Services (RESULTS) Act offers a pathway to correct these flaws and stabilize laboratory reimbursement.
PAMA vs. RESULTS Act
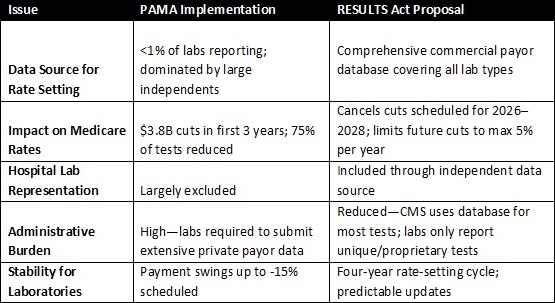
Bottom line: The RESULTS Act addresses the systemic flaws of PAMA by broadening data sources, reducing administrative burden, and preventing destabilizing cuts to lab reimbursement.
The Problem with PAMA
PAMA was designed to align Medicare’s Clinical Laboratory Fee Schedule (CLFS) with private market rates. However, because the initial data collection excluded most hospital outreach labs and physician office labs, the rates reflected primarily the contracts of large national labs. These labs often accept below Medicare reimbursement to secure high volume contracts with insurers and push competitors out of network.
The result: Medicare rates were artificially suppressed, leading to $3.8 billion in cuts between 2017 and 2020 and ongoing payment instability. Smaller community labs and hospital outreach programs, essential for timely, local patient care, have been disproportionately harmed.
National Insurers and Market Distortion
Large payors such as United Healthcare, Cigna and Aetna further exacerbate the issue. By contracting with just one or two national labs, they can claim 'network adequacy' while excluding smaller independent or hospital-based labs. This dynamic undermines competition, reduces patient access to local testing options, and accelerates consolidation in the laboratory sector.
How the RESULTS Act Provides a Fix
The RESULTS Act, with bipartisan support in both chambers of Congress, introduces several key reforms:
• Comprehensive Data Sources: Uses independent commercial payor databases to capture all segments of the lab market, not just large independents
• Cut Prevention: Cancels scheduled cuts of up to 15% in 2026–2028 and caps future annual reductions at 5%
• Reduced Burden: Shifts most data collection away from labs, except for proprietary and Advanced Diagnostic Laboratory Tests (ADLTs)
• Payment Stability: Freezes CLFS rates at 2025 levels until new methodology begins in 2029
• Innovation Support: Protects reimbursement needed for labs to invest in next-generation diagnostic technologies.
Why It Matters for Clinical Laboratories
Without reform, labs face ongoing cuts that threaten their financial stability, limit investment in new testing technologies, and reduce access for patients, particularly in rural and underserved areas. The RESULTS Act offers long-term stability, fairer representation of all lab types, and a pathway to sustainable reimbursement.
Conclusion
The RESULTS Act is not just a technical correction, it is a lifeline for clinical laboratories and the patients they serve. By fixing the flaws in PAMA’s implementation, Congress can ensure fair Medicare reimbursement, reduce administrative burden, and protect the nation’s diagnostic infrastructure.
APS Medical Billing will continue to monitor developments around the RESULTS Act and keep laboratory leaders informed as the policy debate advances.

